Instruments
Bio-Rad Bio-Plex flow cytometer
The Bio-Rad Bio-Plex flow cytometer is specially designed to analyze microsphere based multiplex protein assays. This is an alternative to using ELISA assays to determine the concentration of proteins in solution, such as cell lysates. The advantage of doing multiplex assays is that one can get results simultaneously for many proteins dissolved in a very small volume. Using the user-friendly Bio-Plex Manager software, standard curves of known concentration of all analytes are automatically computed and all data from the standards and unknowns can be displayed in graphic or table form. The results can also be saved in either form whichever is preferred by the investigator. Kits for simultaneous quantitative measurement of up to 25-30 proteins are available. Examples of proteins that can be analyzed with commercially prepared Luminex microspheres are human/murine/rat cytokines, human/murine phosphoproteins, growth factors, kinases, and transcription factors. Users may also create custom assays for any proteins having corresponding antibodies.
This cytometer is available for self-run by investigators who have been trained by the flow cytometry core lab faculty OR who desire to have their samples processed by FCC staff. For investigators who have been trained to run samples themselves, samples need to be prepared in their lab and plates can be brought to the FCC to be run on the Bioplex instrument. Investigators who wish to have their samples run by FCC staff are advised to consult with FCC staff in advance to discuss their experiments and schedule appointments.
Wash stations and hand-held magnets are available to help in the washing of bioplex plates for both magnetic and non-magnetic beads (see parts).
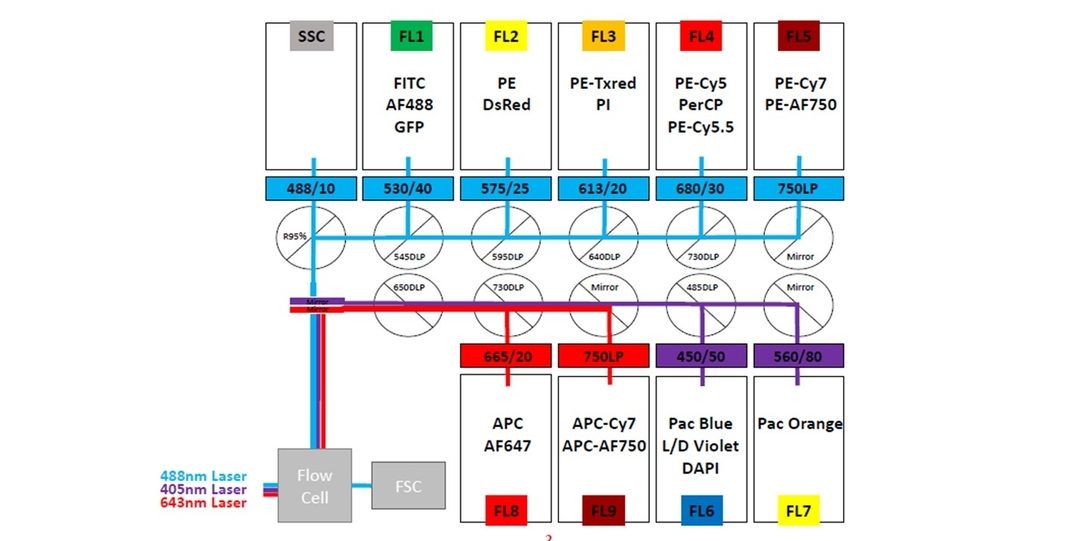
CyAn 1 bench-top analyzer
The CyAn ADP Analyzer is a 3-laser cytometer with Advanced Digital Processing, state-of-the-art optics, and electronics and Easy to walk-up operation. CyAn I has 9 parameters (FSC, SCC, FL1-FL9) and is equipped with 405nm, 488 nm and 642 nm solid-state lasers. This cytometer is available for self-run by investigators who have been trained by the flow cytometry core lab faculty only. Biohazardous samples must not be run on the CyAn. Any potentially biohazardous sample must be fixed.
The instrument is located as a satellite facility in Room 817, Medical Sciences building, 835, S Wolcott Avenue, Chicago, IL 60612.
CyAn 2 bench-top analyzer
CyAn 2 bench-top analyzer
The CyAn2 Analyzer is a 3-laser cytometer with Advanced Digital Processing, state-of-the-art optics and electronics and Easy to walk-up operation. CyAn2 has 9 parameters (FSC, SCC, FL1-FL9) and is equipped with 405nm, 488 nm and 642 nm solid-state lasers. This cytometer is available for self-run by investigators who have been trained by the flow cytometry core lab faculty only. Investigators who require assisted acquisition of samples can talk to the flow cytometry staff to receive permission to enable to make reservations for assisted acquisition. First preference will be given to users who require assisted acquisition. Biohazardous samples must not be run on the CyAn. Any potentially biohazardous sample must be fixed.
The instrument is located at E-25 (in the basement), Medical Sciences building, 835 S Wolcott Avenue, Chicago, IL 60612.
CyTOF Mass cytometer
Mass cytometry is a novel technology that combines some aspects of flow cytometry and with time-of-flight mass spectrometry. This technology utilizes metal-labeled antibody tags, instead of fluorophores used in traditional flow cytometry, and as each elemental mass channel is distinct, requires minimal or no signal compensation. Importantly, the single cell resolution of flow cytometry is retained, and with the current set up around 32 parameters can be measured using the CyTOF. The CyTOF thus permits the study of complex biological systems at the single-cell level.
Gallios bench-top analyzer
The Gallios flow cytometer provides efficient acquisition of excellent quality data from up to 10 colors with advanced optical design for enhanced sensitivity for multicolor assays. The multi-tube carousel loader provides quick and easy setup of automated walk-away processing of the samples. The cytometer carries 3 solid state diode lasers (488nm Blue, 638nm Red & 405nm Violet) and can analyze up to 10 colors (5 + 3 + 2). The Gallios also has a unique FSC detector that improves resolution of small particles.
The FCC provides services for acquiring samples for investigators who require such assistance.
Samples should be processed in the labs and should be brought to the FCC in BD Falcon 5 ml round bottom polystyrene tubes (12x75mm style) or equivalents. Cells must be filtered by passing through cell strainers prior to being submitted to the FCC. Samples that are sticky or show the presence of clogs will be filtered by FCC staff using cell strainers from the FCC and the investigating PI will be charged for the cost of the tubes. Users will be required to fill in a sample submission sheet with details of the samples and the fluorophores at the time of submission. It is the responsibility of the investigator to insure that the fluorophores used in the experiment can be run on the cytometer.
The rate at which samples are run are dependent on the quality of sample prep, total number of events to be acquired and the kind of assay. Please determine the time required based on the above factors. On an average, allow for 15-20 samples an hour if you are acquiring 20,000 events per sample. A pilot experiment may be required to determine more accurately the time for running an experiment.
BD LSRFortessa flow cytometer
The BD LSRFortessa flow cytometer is configured with 4 lasers—blue, red, violet, and yellow-green which enables the detection of up to 13 colors simultaneously. Arranged in octagon- and trigon-shaped optical pathways, their novel design efficiently maximizes signal detection and increases sensitivity and resolution. This allows researchers to identify cells, especially dim and rare cell populations, optimizing multicolor assays and panel design for superior results.
The LSR Fortessa provides efficient acquisition of excellent quality data from up to 13 colors with advanced optical design for enhanced sensitivity for multicolor assays. The instrument is also equipped with a High Through put plate reader that helps run 96-well and 384-well plates and provides for rapid, fully automated sample acquisition from microtiter plates. This provides easier processing of the samples. The cytometer carries 4 solid state diode lasers (488nm Blue, 561nm Yellow 638nm Red & 405nm Violet,) and can analyze up to 13 colors (2+5 +3 + 3).
The FCC provides services for acquiring samples for investigators who require such assistance.
Samples should be processed in the labs and should be brought to the FCC in BD Falcon 5 ml round bottom polystyrene tubes (12x75mm style) or equivalents. Cells must be filtered by passing through cell strainers prior to being submitted to the FCC. Samples that are sticky or show the presence of clogs will be filtered by FCC staff using cell strainers from the FCC and the investigating PI will be charged for the cost of the tubes. Users will be required to fill in a sample submission sheet with details of the samples and the fluorophores at the time of submission. It is the responsibility of the investigator to insure that the fluorophores used in the experiment can be run on the cytometer.
The rate at which samples are run are dependent on the quality of sample prep, total number of events to be acquired and the kind of assay. Please determine the time required based on the above factors. On an average, allow for 15-20 samples an hour if you are acquiring 20,000 events per sample. A pilot experiment may be required to determine more accurately the time for running an experiment.
MoFlo Astrios cell sorter
The MoFlo Astrios is a high speed cell sorter with 5 lasers capable of 6-way sorting. It is equipped with 355nm, 405nm, 488nm, 561nm and 640nm lasers. The sorter is placed inside a Baker bio safety cabinet for sorting bio safety level two samples. Events can be sorted into different collection devices including microscope slides, micro tubes, 12×75 test tubes, 15ml or 50ml falcon tubes, as well as multi-well plates.
Reservations are made by calling 312-996-2775 (6-2775 from within UIC campus). You will need to provided information about the fluorochromes you are using for you assay, along with the cell number, the kind of collection tubes you will collect sorted samples into and any specific needs for your assay. You are strongly encouraged to discuss your assay with the Director of the facility prior to preparing your experiments for sort.
All sorts include a sort set up charge and run time based on scheduled and actual run. Cancelations must be made within 48 hours of run time to not incur scheduled price charges. Final decisions to choose the appropriate cell sorter for the samples will rest with the flow cytometry staff based on requirement and availability.
Work station
The analysis workstation will be available for investigator who have run their samples in the FCC only and who have been trained in software analysis by FCC staff. will The workstation carries different analysis software to analyze flow cytometry data. Training will be provided to the investigators (see Analysis training under services) to use different software based on their needs.
The different software on the Workstation includes Summit, FlowJo, Kaluza, ModFit and WinMDI.